Geevo αβ valve (split butterfly valve) Pass OEB5 containment performance grade test successfully!
Recently, our produced αβ valve( split butterfly valve) has successfully passed the OEB5 containment performance grade test by the Third party qualification institute!
Geevo company pays high attention to the research and manufacture on αβ valve (split butterfly valve) product, having independent intellectual property rights (1 invention patent and multiple utility model patents), in order to meet the demands from users, after multiple communications with users with different needs and preparation of testing plans, finally three sets of different types of alpha beta valve were prepared for containment performance testing. A third-party company was commissioned to conduct on-site equipment sealing evaluation on these three sets of alpha beta valves to determine their CPT (containment performance target) and OEB (occupational exposure Band) that can be used for safe operation of active pharmaceutical ingredients.
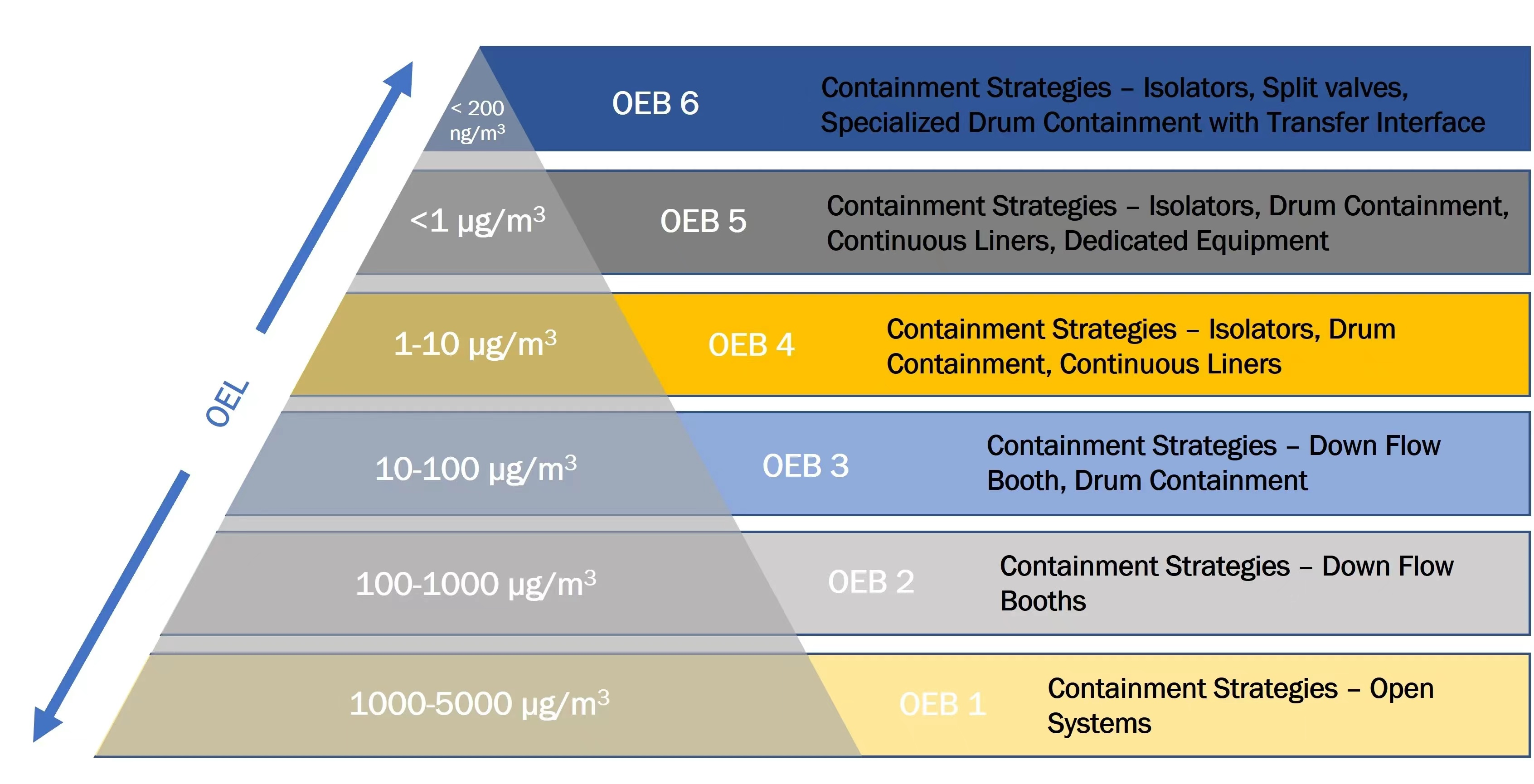
OEB (Occupational Exposure Band), substances with different toxicity and biological activity are classified into OEB1-5 levels from high to low based on the allowable dust concentration range in the working environment.
Equipment requirements for handling OEB5 grade samples: When operating a certain amount of OEB5 grade substances inside the equipment, the retention concentration in the surrounding air shall not exceed 1μg/m3 or lower (determined by the sealing target value CPT provided by the equipment manufacturer).
The containment performance targets(CPTs) for these three set αβ valve were set as follows:
◎ The CPT of two set αβ valve with CIP/SIP type are set at 0.1μg/m3 suitable for handling active pharmaceutical ingredients with an occupational exposure limit (OEL) 0.1μg/m3(classified as OEB5).
◎ The CPT of one set αβ valve with conventional type is set at 1μg/m3 suitable for handling active pharmaceutical ingredients with an occupational exposure limit (OEL) 1μg/m3(classified as OEB4).
The method of containment performance assessment of this project refers to the containment performance assessment of particulate matter in pharmaceutical industry equipment , recorded in ISPE Good Practice Guide, which was developed by SMFEPAC. Lactose was used as the surrogate to simulate the operation of transferring materials through the αβ valve from the stainless steel container, to assessment the containment performance of the αβ valve.
The samples are prepared and analyzed by Safebridge Laboratory in USA. The laboratory’s internal method specifies a lactose quantification detection limit of 0.5ng/filter.


Testing site photo
Based on on-site observation and substitute lactose detection:
All substitute (lactose) fixed-point testing and the air concentration exposed to by operators are significantly lower than the CIP/SIP type containment performance targets values (CPT, 0.1μg/m3) and conventional type containment performance targets values (CPT, 1μg/m3) set by our company.
CIP/SIP Type
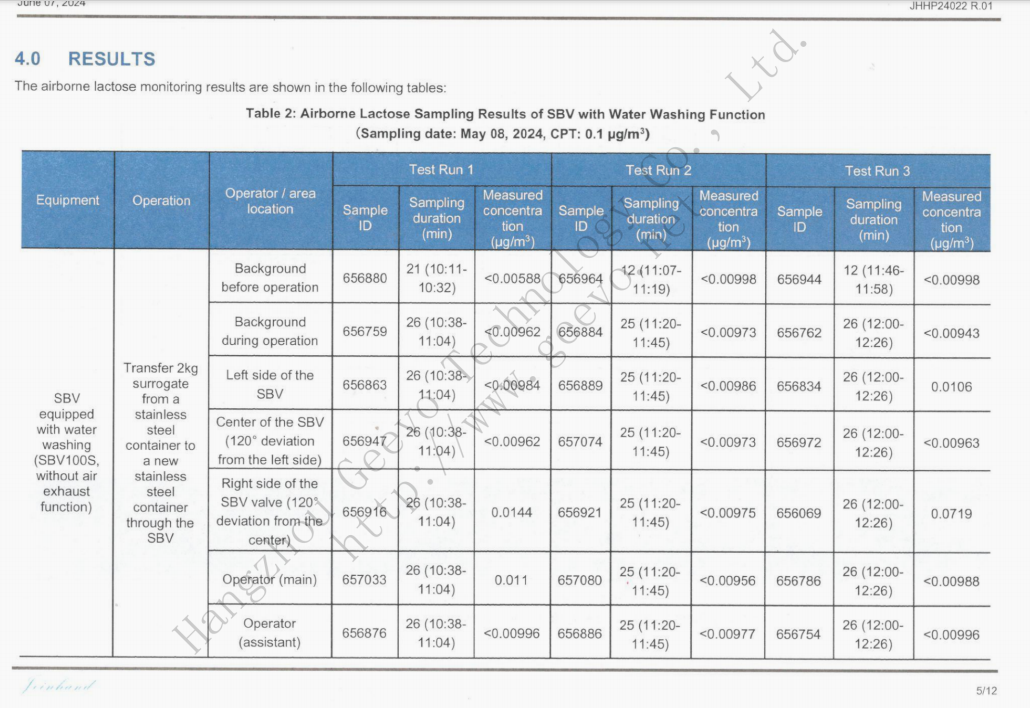
Summary of Detected Data
The test result indicates that:
During the process of transferring substitutes through three types of αβ valve of Geevo, the detected substitute air detection results can meet the CPT (0.1μg/m3 and 1μg/m3) operation requirements of Geevo.

Regarding of the structural and usage characteristics of this product, please refer to the product introduction or contact our sales personnel directly.
Invention Patent Number: 2016103499241
Utility model patent number: 2016204838046、2023205790256、2023205790275、2023205359316